PROTOCOL - RNA DNA extractions from coral larvae (Zymo)
This protocol details the concurrent extraction of RNA and DNA using the Zymo Quick-DNA/RNA Miniprep Plus Kit on planulae (larvae) and newly settled individuals (spat) of the coral Stylophora pistillata.
Following tests of this kit by others e.g. Kevin Wong, Erin Chille, Federica Scucchia I worked with 20 planulae in each vial or 6 - 8 spat in each vial. 700 uL of RNA DNA Shield was added to each sample upon collection. Samples were stored at -20 degrees until processing.
Reagents preparation
- Add 96 mL 100% ethanol (104 mL 95% ethanol) to the 24 mL DNA/RNA Wash Buffer concentrate before use. DNA/RNA Wash Buffer included with D7003T (Mini Prep Plus Kit) is supplied ready-to-use and does not require the addition of ethanol prior to use. Check kit contents and instructions to confirm prep steps.
- Reconstitute the lyophilized (freeze-dried) DNase I as indicated on the vial prior to use. Mix by inversion. Store frozen aliquots.
- Prepare the 10 mM Tris HCl following this protocol.
Sample Preparation
Adapted From E. Chille’s Protocol with the addition of bead beating:
- To each sample tube, add 5 – 10 2 mm beads
- Shake for 60secs horizontally in bead beater. After shaking, allow the sample liquid to settle for few minutes.
- Remove 300 uL sample to new labelled tube. Keep rest of the sample as spare in the fridge.
- To the 300 ul sample, add 30 µl of PK digestion buffer
- Add 15 µl Proteinase K to each sample tube
- Vortex tubes to mix and hold at room temperature for 30 mins
- Add equal volume (345 µl) DNA/RNA lysis buffer to each sample tube
- Finger flick to mix tubes
- Centrifuge at 16,000 rcf (g) for 30 seconds
DNA Extraction
- Set up yellow DNA spin columns and collection tubes, label appropriately
- Warm elution liquids to 70 degrees °C (10mM Tris HCl pH. 8.0 and RNase free water)
- Add 690 µl (total volume) of sample gently to the yellow DNA spin column
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Important Save the flow through from this step: transfer to a new 1.5mL tube labelled for RNA. Move to the RNA protocol to compete DNase treatment (steps 1 – 14)
- Add 400µl DNA/RNA Prep Buffer gently to the yellow DNA spin columns
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Add 700 µl DNA/RNA Wash Buffer gently to the yellow DNA spin columns
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Add 400 µl DNA/RNA Wash Buffer genetly to the yellow DNA spin columns
- Centrifuge at 16,000 rcf (g) for 2 minutes
- Discard flow through (Zymo kit waste)
- Transfer yellow columns to new 1.5mL microcentrifuge tubes
- Add 50 µl warmed 10 mM Tris HCl to each yellow DNA column by dripping slowly directly on the filer
- Incubate at room temp for 5 minutes
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Repeat steps 18-20 for a final elution volume of 100 µl. If concentrations are low, elute once in 50 uL total volume.
- Label tubes, store at 4 °C if quantifying the same day or the next, if waiting longer store in -20 °C
RNA Extraction
Can do concurrently with DNA Extraction after DNA Extraction Step 7
- Add equal volume (700 µl) 100% EtOH to the 1.5mL tubes labelled for RNA containing the original yellow column flow through
- Vortex and spin down to mix
- Add 700 µl of that liquid to the green RNA spin columns
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Add 700 µl to the green RNA spin columns (the rest from the 1.5mL RNA tubes)
- Centrifuge at 16,000 rcf (g) for 30 seconds o Get DNase I from freezer
- Discard flow through (Zymo kit waste)
- Add 400 µl DNA/RNA Wash Buffer gently to each green RNA column
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Make DNase I treatment master mix: o 75µl DNA Digestion buffer x # of samples o 5µl DNase I x # of samples
- Add 80 µl DNase I treatment master mix directly to the filter of the green RNA columns
- Incubate at room temp for 15 minutes
From here: combine steps with the DNA samples
- Add 400 µl DNA/RNA Prep Buffer gently to each column
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Add 700 µl DNA/RNA Wash Buffer gently to the green RNA spin columns
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Discard flow through (Zymo kit waste)
- Add 400 µl DNA/RNA Wash Buffer genetly to the green RNA spin columns
- Centrifuge at 16,000 rcf (g) for 2 minutes
- Discard flow through (Zymo kit waste)
- Transfer green columns to new 1.5mL microcentrifuge tubes
- Add 50µl warmed DNase/RNase free water to each green RNA column by dripping slowly directly on the filer
- Incubate at room temp for 5 minutes
- Centrifuge at 16,000 rcf (g) for 30 seconds
- Repeat steps 25-27 for a final elution volume of 100µl. If concentrations are low, elute once in 50 uL total volume.
- Aliquot 5 – 10 µl to save for Nanodrop and Tape Station to avoid freeze-thaw of your stock sample
- Store all tubes in the -80 °C
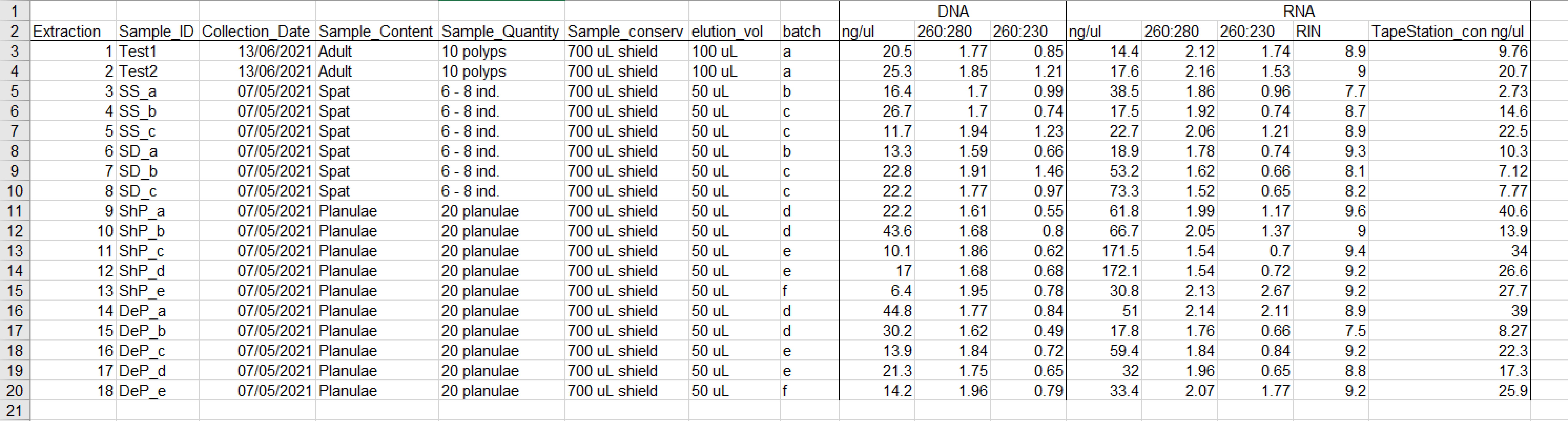
Check the DNA and RNA quality on the NanoDrop and Tape Station
.jpg)
.jpg)
.jpg)